Chemical Structures – Bonding
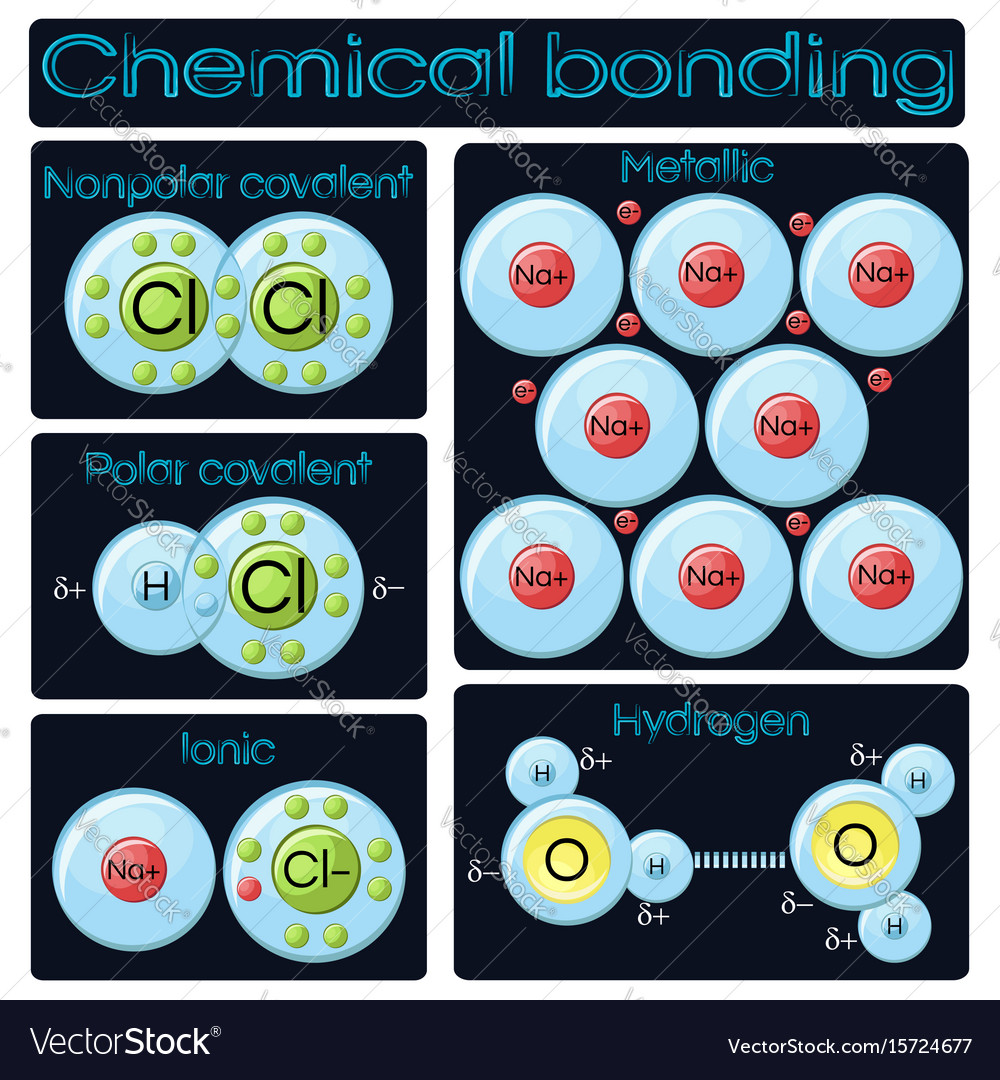
Bonding is the process by which atoms combine to form molecules or compounds. There are different types of chemical bonds, including covalent bonds, ionic bonds, and metallic bonds.
Covalent bonds occur when atoms share electrons to form a stable molecule. Covalent bonds can be polar or nonpolar, depending on the electronegativity difference between the atoms involved in the bond. In a nonpolar covalent bond, the electrons are shared equally between the atoms, while in a polar covalent bond, the electrons are shared unequally, resulting in a partial positive and partial negative charge on the atoms.
Ionic bonds occur when there is a complete transfer of electrons from one atom to another, resulting in the formation of ions. In an ionic bond, one atom loses electrons to become a cation (positively charged ion), while the other atom gains electrons to become an anion (negatively charged ion). The resulting ions are held together by electrostatic attractions between opposite charges.
Metallic bonds occur in metals and involve the sharing of electrons among a lattice of metal atoms. Metallic bonding is characterized by a delocalized cloud of electrons that can move freely throughout the lattice, allowing metals to conduct electricity and heat.
In addition to these three main types of bonds, there are also hydrogen bonds, van der Waals forces, and other weak intermolecular forces that can contribute to the overall stability and properties of molecules.
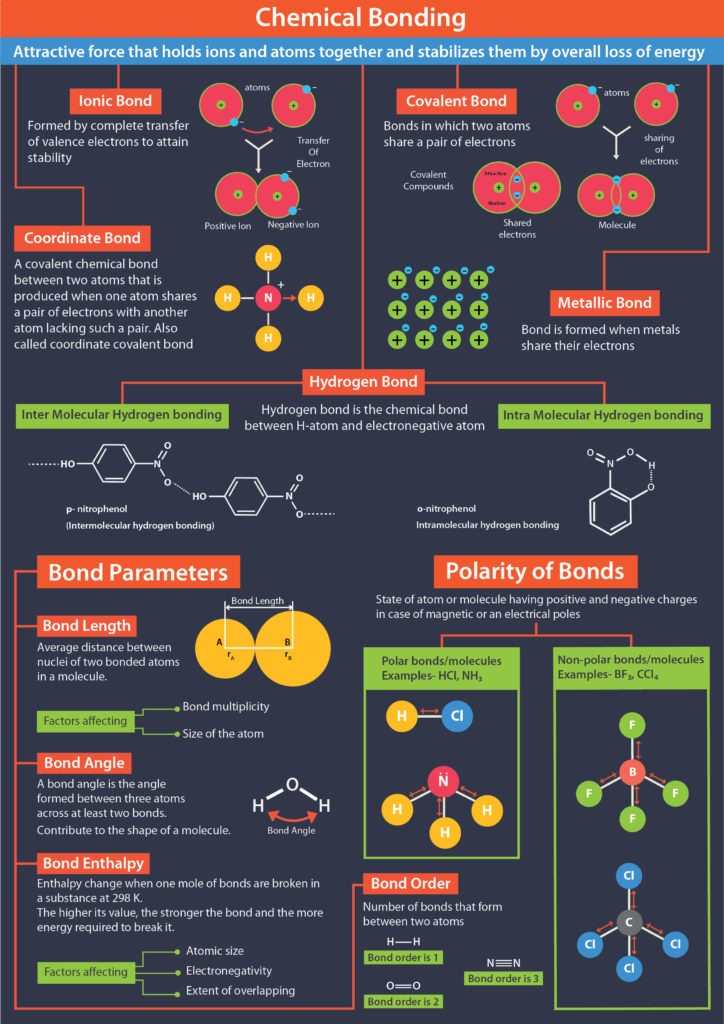
Understanding the type of bonding in a molecule is important for predicting its properties, such as its melting and boiling points, solubility, and reactivity. For example, covalent molecules with strong polar bonds tend to have higher boiling points than those with weaker nonpolar bonds, while ionic compounds tend to have high melting and boiling points due to the strong electrostatic interactions between the ions.
