Chemical Structures – Molecular Geometry
Chemical Structures – Molecular Geometry
Chemical structures are not only about the arrangement of atoms and electrons in a molecule, but also about the way in which these atoms are arranged in three-dimensional space. Molecular geometry refers to the three-dimensional shape of a molecule, which is determined by the arrangement of its atoms and the nature of its chemical bonds.
The shape of a molecule is important because it can influence its physical and chemical properties. For example, the polarity of a molecule, its reactivity, and its solubility can all be influenced by its molecular geometry.
The steps to determine the molecular geometry of a molecule are as follows:
- Draw the Lewis structure of the molecule.
- Determine the number of electron pairs (bonding and non-bonding) around the central atom.
- Determine the electron pair geometry of the molecule by considering all electron pairs, both bonding and non-bonding. This can be done using the VSEPR (Valence Shell Electron Pair Repulsion) theory.
- Determine the molecular geometry of the molecule by considering only the bonding electron pairs. This can be done by taking into account the repulsion between the electron pairs and the effect of lone pairs of electrons.
- Draw a three-dimensional representation of the molecule, taking into account its molecular geometry.
For example, the molecular geometry of carbon dioxide (CO2) can be determined as follows:
- The Lewis structure of CO2 shows that it has two double bonds between the carbon atom and the two oxygen atoms.
- The number of electron pairs around the carbon atom is four, which gives it a tetrahedral electron pair geometry.
- However, since both the oxygen atoms are double-bonded to the carbon atom and do not have any lone pairs of electrons, the molecular geometry of CO2 is linear.
- The three-dimensional representation of CO2 shows that it is a linear molecule, with the carbon atom in the center and the two oxygen atoms on either side.
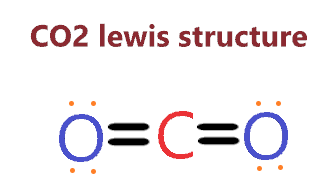
Electron Pair Geometry: The electron pair geometry of a molecule is determined by the arrangement of all electron pairs, both bonding and non-bonding, around the central atom. This can be determined using the VSEPR theory, which states that electron pairs in the valence shell of an atom repel each other and try to stay as far apart as possible. The most common electron pair geometries are linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral.
Molecular Geometry: The molecular geometry of a molecule is determined by the arrangement of only the bonding electron pairs around the central atom. This is because lone pairs of electrons take up more space than bonding pairs and can distort the molecular geometry. For example, a molecule with a tetrahedral electron pair geometry can have a trigonal pyramidal molecular geometry if it has one lone pair of electrons.
Bond Angles: The bond angles in a molecule are determined by its molecular geometry. The bond angle is the angle between two adjacent bonds, measured from the centre of the central atom. The bond angle can be influenced by the presence of lone pairs of electrons, which can cause bonding pairs of electrons to be pushed closer together.
Polarity: The molecular geometry of a molecule can influence its polarity, which is a measure of the separation of electrical charges within the molecule. A polar molecule has a positive and negative end, while a nonpolar molecule does not. The polarity of a molecule is determined by the shape of the molecule and the polarity of its individual bonds.
Symmetry: The molecular geometry of a molecule can also determine its symmetry. A molecule is symmetric if it can be divided into two identical halves by a plane or a line. Symmetric molecules can have different physical and chemical properties compared to asymmetric molecules.
Overall, understanding molecular geometry is important for predicting the properties and behaviour of molecules. It can also be useful for designing new molecules with specific properties, such as drugs or catalysts.
