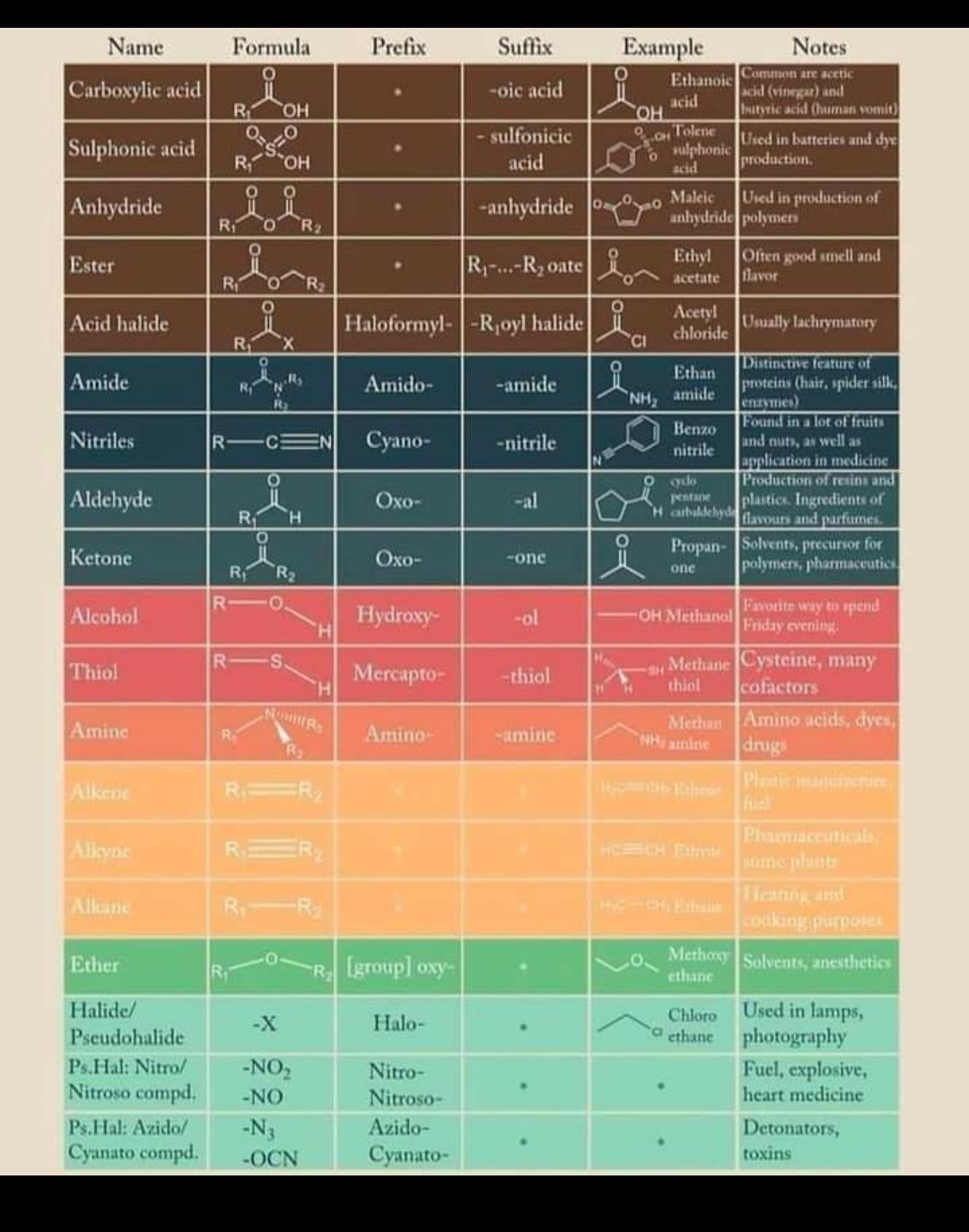
IUPAC Nomenclature of Organic Compounds

The International Union of Pure and Applied Chemistry (IUPAC) nomenclature system is used to name organic compounds systematically based on their chemical structure. The IUPAC nomenclature system is designed to provide a unique and unambiguous name for each compound, which can be easily understood by chemists all over the world.
The basic rules of the IUPAC nomenclature system include the following:
- Identify the longest carbon chain: The first step in naming an organic compound is to identify the longest continuous chain of carbon atoms in the molecule. This chain is known as the parent chain.
- Number the carbon atoms: The carbon atoms in the parent chain are numbered sequentially, starting from the end that gives the lowest possible number to the substituents.
- Identify the substituents: Any groups or atoms that are attached to the parent chain are called substituents. Substituents are named based on their functional groups, such as alkyl, halogen, or hydroxyl groups.
- Assign locants to the substituents: The locant is the number assigned to each substituent to indicate the position of the substituent on the parent chain. The locant is placed before the name of the substituent.
- Arrange the names alphabetically: The names of the substituents are arranged alphabetically, ignoring any prefixes such as di-, tri-, tetra-, etc.
- Use prefixes to indicate the number of substituents: When there is more than one substituent of the same type, prefixes such as di-, tri-, tetra-, etc., are used to indicate the number of substituents.
- Indicate the position of functional groups: Functional groups are indicated by using suffixes such as -ol for alcohols, -one for ketones, -al for aldehydes, -oic acid for carboxylic acids, and -amine for amines. The suffix is added to the end of the parent chain name.
- Use parentheses to indicate branching: When there are branching chains, parentheses are used to enclose the name of the branch.
- Use commas to separate numbers: When there are more than one number, they are separated by commas.
- Use prefixes for cyclic compounds: Cyclic compounds are named by indicating the number of carbon atoms in the ring, followed by the prefix “cyclo-“. The carbon atoms in the ring are numbered starting from any carbon atom and proceeding in either direction around the ring to give the lowest possible locants to the substituents.
- Use prefixes for multiple bonds: If the molecule contains a double bond, the suffix “-ene” is added to the name of the parent chain. The position of the double bond is indicated by the lowest possible number of the two carbons that are involved in the double bond. Similarly, if there is a triple bond, the suffix “-yne” is added to the parent chain name.
- Use prefixes for functional groups: Some functional groups require prefixes before the suffix. For example, if there are two hydroxyl groups, the prefix “diol” is used before the “-ol” suffix. Similarly, if there are two carbonyl groups, the prefix “dione” is used before the “-one” suffix.
- Consider stereochemistry: Stereochemistry is the study of the three-dimensional arrangement of atoms in molecules. Stereoisomers are molecules that have the same molecular formula and connectivity, but differ in their spatial arrangement of atoms. In IUPAC nomenclature, stereochemistry can be indicated by using prefixes such as “cis-” or “trans-” for geometric isomers, or “R-” or “S-” for enantiomers.
- Use brackets for complex substituents: Some substituents can be complex, containing their own branches and functional groups. In these cases, the complex substituent is enclosed in brackets and assigned its own locant.
- Use parentheses to indicate functional groups on a side chain: If there is a functional group on a side chain, parentheses are used to enclose the name of the side chain, and the functional group suffix is added to the end of the side chain name.
Overall, the IUPAC nomenclature system is a standardized and systematic way of naming organic compounds, which ensures that each compound has a unique and unambiguous name. While it can be complex and involve a lot of rules, understanding IUPAC nomenclature is essential for communication among chemists and for a deeper understanding of organic chemistry.
IUPAC Name Chart for quick Reference :