Chemical Structures – Hybridization
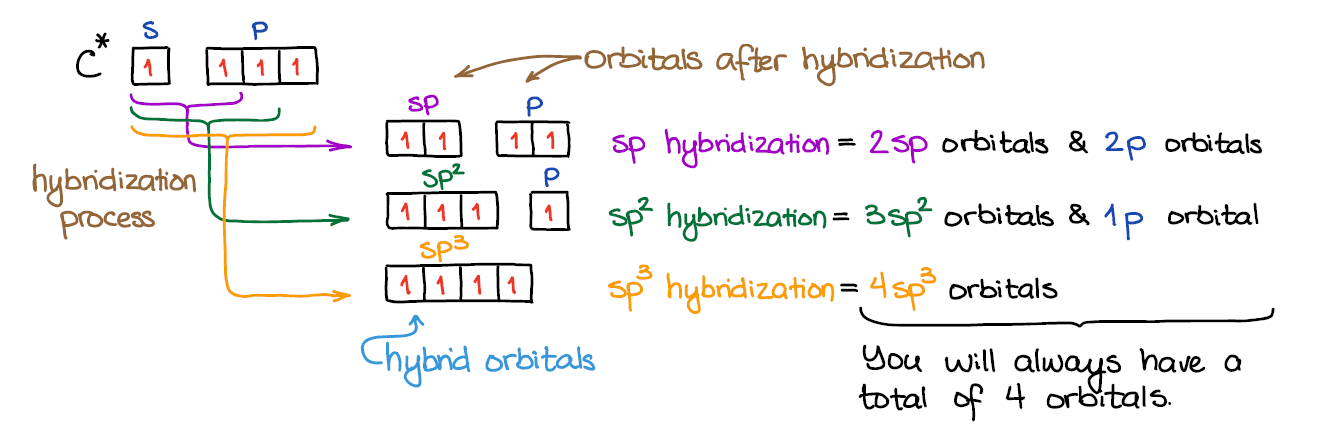
Hybridization is a concept used in chemistry to describe the mixing of atomic orbitals to form new hybrid orbitals that can better explain the geometry and bonding of molecules.
The most common types of hybridization are sp, sp2, and sp3 hybridization, which involve the combination of s and p orbitals.
- sp hybridization occurs when one’s orbital and one p orbital combine to form two sp hybrid orbitals. These hybrid orbitals are linear in shape and oriented at 180 degrees to each other. Examples of molecules with sp hybridization include acetylene (C2H2) and carbon monoxide (CO).

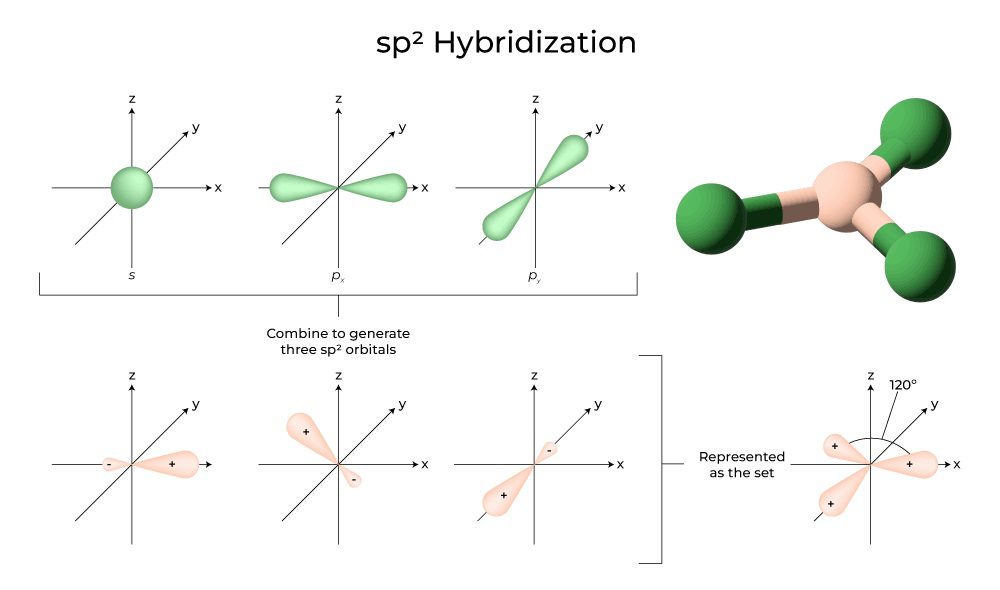
- sp2 hybridization occurs when one’s orbital and two p orbitals combine to form three sp2 hybrid orbitals. These hybrid orbitals are trigonal planar in shape and oriented at 120 degrees to each other. The unhybridized p orbital is perpendicular to the plane of the sp2 hybrid orbitals. Examples of molecules with sp2 hybridization include ethylene (C2H4) and formaldehyde (CH2O).
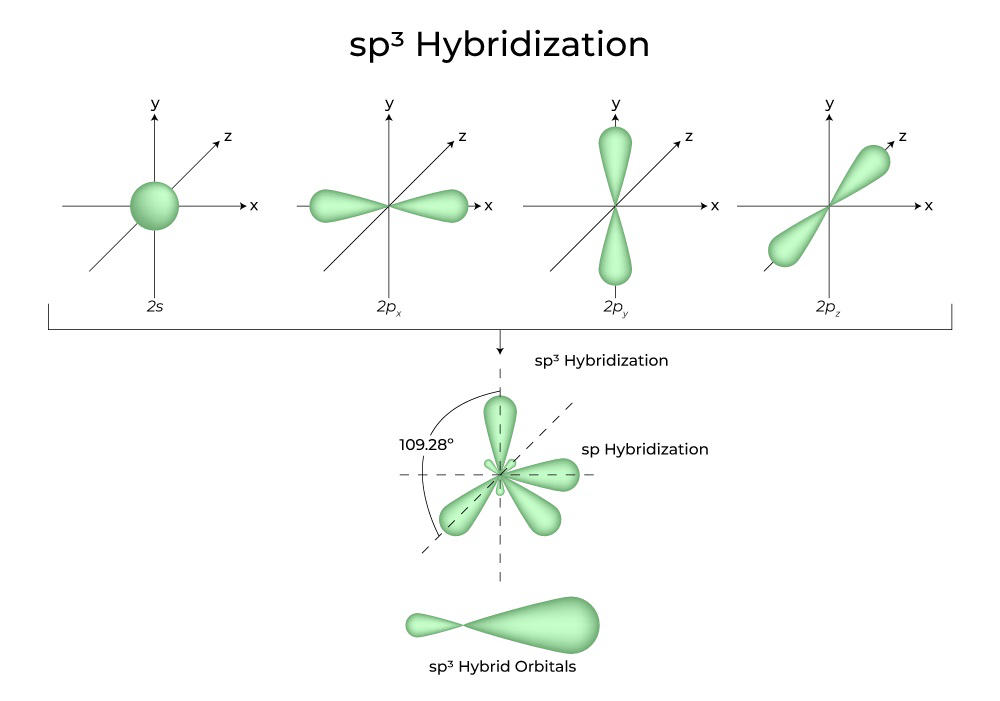
- sp3 hybridization occurs when one s orbital and three p orbitals combine to form four sp3 hybrid orbitals. These hybrid orbitals are tetrahedral in shape and oriented at 109.5 degrees to each other. Examples of molecules with sp3 hybridization include methane (CH4) and ammonia (NH3).
These hybrid orbitals allow for the formation of stronger and more stable bonds than what would be possible with just pure atomic orbitals. The concept of hybridization is important in understanding the geometry, polarity, and reactivity of molecules.
In addition to sp, sp2, and sp3 hybridization, there are also other types of hybridization that are less common, such as sp3d, sp3d2, and sp3d3 hybridization.
- sp3d hybridization occurs when one’s orbital, three p orbitals, and one d orbital combine to form five sp3d hybrid orbitals. These hybrid orbitals are trigonal bipyramidal in shape and oriented at 90 and 120 degrees to each other. Examples of molecules with sp3d hybridization include phosphorus pentafluoride (PF5) and sulphur-hexafluoride (SF6).
- sp3d2 hybridization occurs when one’s orbital, three p orbitals, and two d orbitals combine to form six sp3d2 hybrid orbitals. These hybrid orbitals are octahedral in shape and oriented at 90 degrees to each other. Examples of molecules with sp3d2 hybridization include sulphur tetrafluoride (SF4) and xenon hexafluoride (XeF6).
- sp3d3 hybridization occurs when one’s orbital, three p orbitals, and three d orbitals combine to form seven sp3d3 hybrid orbitals. These hybrid orbitals are pentagonal bipyramidal in shape and oriented at 90 and 72 degrees to each other. Examples of molecules with sp3d3 hybridization include iodine heptafluoride (IF7).
The concept of hybridization is important in predicting the geometry of molecules, which is crucial for understanding their properties and reactivity. For example, the angle between hybrid orbitals can affect the strength and directionality of bonds, as well as the polarity of a molecule. In addition, the type of hybridization can also affect the hybridization energy, which is the energy required to form hybrid orbitals.
