Inductive Effects, Electromeric Effects, Resonance & Hyper Conjugation
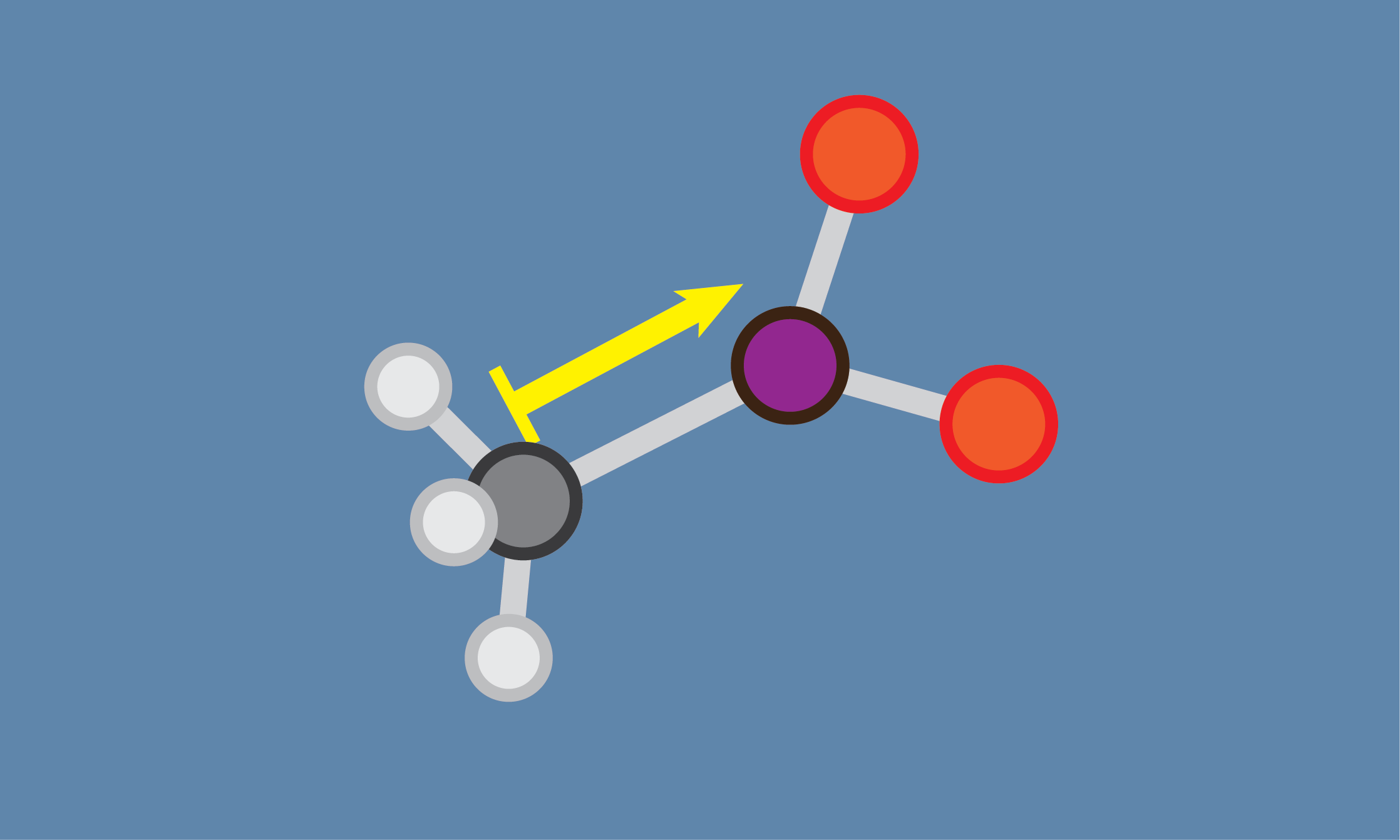
Inductive Effect in Chemistry
Inductive effect is a type of electronic effect that describes the distribution of electron density in a molecule. It occurs when a polar covalent bond between two atoms causes a shift in the distribution of electrons in the molecule. This shift can affect the reactivity and stability of the molecule.
The inductive effect is based on the difference in electronegativity between two atoms in a molecule. Electronegativity is a measure of an atom’s ability to attract electrons to itself in a chemical bond. When two atoms in a molecule have different electronegativities, the electrons in the bond are not shared equally. The more electronegative atom will attract the electrons more strongly, creating a partial negative charge, while the less electronegative atom will have a partial positive charge.
This polarization of the electron density in a bond creates an inductive effect that can affect nearby atoms or groups in the molecule. The inductive effect can either increase or decrease the electron density in a molecule or functional group, depending on the nature of the polar bond.
The inductive effect can be described as either +I or -I, depending on whether the polar bond in question causes an increase or decrease in electron density in the molecule. For example, a methyl group (CH3) has a +I effect because the carbon atom is less electronegative than the hydrogen atoms, causing the electrons in the C-H bond to be drawn towards the carbon atom and away from the hydrogen atoms. This creates a partial negative charge on the carbon atom and a partial positive charge on the hydrogen atoms.
In contrast, a fluorine atom (F) has a -I effect because it is more electronegative than the carbon atom to which it is bonded. This causes the electrons in the C-F bond to be drawn towards the fluorine atom, creating a partial negative charge on the fluorine atom and a partial positive charge on the carbon atom.
The inductive effect can have significant effects on the reactivity of a molecule. For example, the presence of electron-donating groups with a +I effect can increase the nucleophilicity of a molecule, making it more likely to undergo reactions with electrophiles. Conversely, electron-withdrawing groups with a -I effect can decrease the nucleophilicity of a molecule, making it less likely to undergo reactions.
In summary, the inductive effect is a type of electronic effect that describes the distribution of electron density in a molecule due to the presence of polar covalent bonds. The effect can either increase or decrease electron density in a molecule, and can affect the reactivity and stability of the molecule.
Electromeric Effect in Chemistry
Electromeric effect is a different type of electronic effect that describes the movement of electrons within a molecule in response to the polarization of a covalent bond.
In the case of the electromeric effect, a bond between two atoms in a molecule becomes polarized due to the presence of a nearby electron-withdrawing or electron-donating group. This polarization causes the electrons in the bond to shift towards the more electronegative atom, resulting in the formation of a partial positive charge on the atom that lost electron density and a partial negative charge on the atom that gained electron density.
The resulting electronic imbalance between the two atoms can lead to the formation of a new bond or the breaking of an existing bond in the molecule. This movement of electrons in response to the polarization of a bond is called the electromeric effect.
The electromeric effect can be either positive or negative depending on whether the bond polarization leads to an increase or decrease in electron density at a particular atom in the molecule. In general, electron-donating groups have a positive electromeric effect, while electron-withdrawing groups have a negative electromeric effect.
For example, consider the case of a carbon-carbon double bond (C=C) in a molecule. If an electron-withdrawing group is present on one side of the double bond, it can polarize the bond and cause the electrons to shift towards the other side of the double bond. This creates a partial negative charge on the carbon atom that gained electron density and a partial positive charge on the carbon atom that lost electron density. This shift in electron density can lead to the formation of a new bond, such as the addition of a nucleophile to the partial positive carbon atom.
In contrast, an electron-donating group on one side of the double bond can polarize the bond and cause the electrons to shift towards the other side of the double bond, creating a partial negative charge on the carbon atom that gained electron density and a partial positive charge on the carbon atom that lost electron density. This shift in electron density can lead to the breaking of the double bond, such as in a reaction with an electrophile.
In summary, the electromeric effect is a type of electronic effect that describes the movement of electrons within a molecule in response to the polarization of a covalent bond. The effect can either increase or decrease electron density at a particular atom in the molecule and can affect the reactivity of the molecule.
Resonance and Hyperconjugation
Resonance and Hyperconjugation are two important concepts in organic chemistry that help explain the stability and reactivity of molecules.
Resonance: Resonance is a phenomenon where a molecule can have multiple valid Lewis structures that can be used to describe its electronic structure. These structures are called resonance structures, and they differ only in the arrangement of electrons. The actual electronic structure of the molecule is a hybrid of these resonance structures, which gives the molecule greater stability and delocalization of electrons.
Resonance structures are indicated by drawing double-headed arrows between them. For example, the benzene molecule can be represented by two resonance structures, where the double bonds are located in different positions:

The actual electronic structure of the benzene molecule is a hybrid of these two resonance structures, which shows that the carbon-carbon bonds are not single bonds or double bonds, but rather have some intermediate character. The delocalization of electrons over the entire ring contributes to the stability of the molecule and its resistance to reactions that would break the ring.
Hyperconjugation: Hyperconjugation is a phenomenon where a sigma (σ) bond between two atoms is involved in the stabilization of a nearby pi (π) bond or a carbocation. The sigma bond donates electron density into the empty π* orbital of the adjacent pi bond or the positively charged carbon atom in the carbocation, which increases the stability of the molecule.
The degree of hyperconjugation depends on the orientation of the sigma bond relative to the pi bond or the carbocation. The most effective hyperconjugation occurs when the sigma bond is in a plane perpendicular to the plane of the pi bond or the carbocation. This arrangement allows maximum overlap of the orbitals and maximizes the electron donation.
For example, in the stability of the tert-butyl carbocation, the adjacent C-H bonds are oriented in such a way that the sigma bond can donate electron density into the empty p orbital of the positively charged carbon atom, which stabilizes the carbocation.
The concept of hyperconjugation also helps to explain the relative stability of substituted alkenes. For example, in the case of 2-methylpropene, the adjacent C-H bonds donate electron density into the adjacent C=C bond, which helps stabilize the double bond and the entire molecule.
In summary, resonance and hyperconjugation are two important concepts in organic chemistry that help explain the stability and reactivity of molecules. Resonance involves the delocalization of electrons in a molecule, while hyperconjugation involves the donation of electron density from a sigma bond to a pi bond or a carbocation.
