Chemical Structures – Lewis Structures
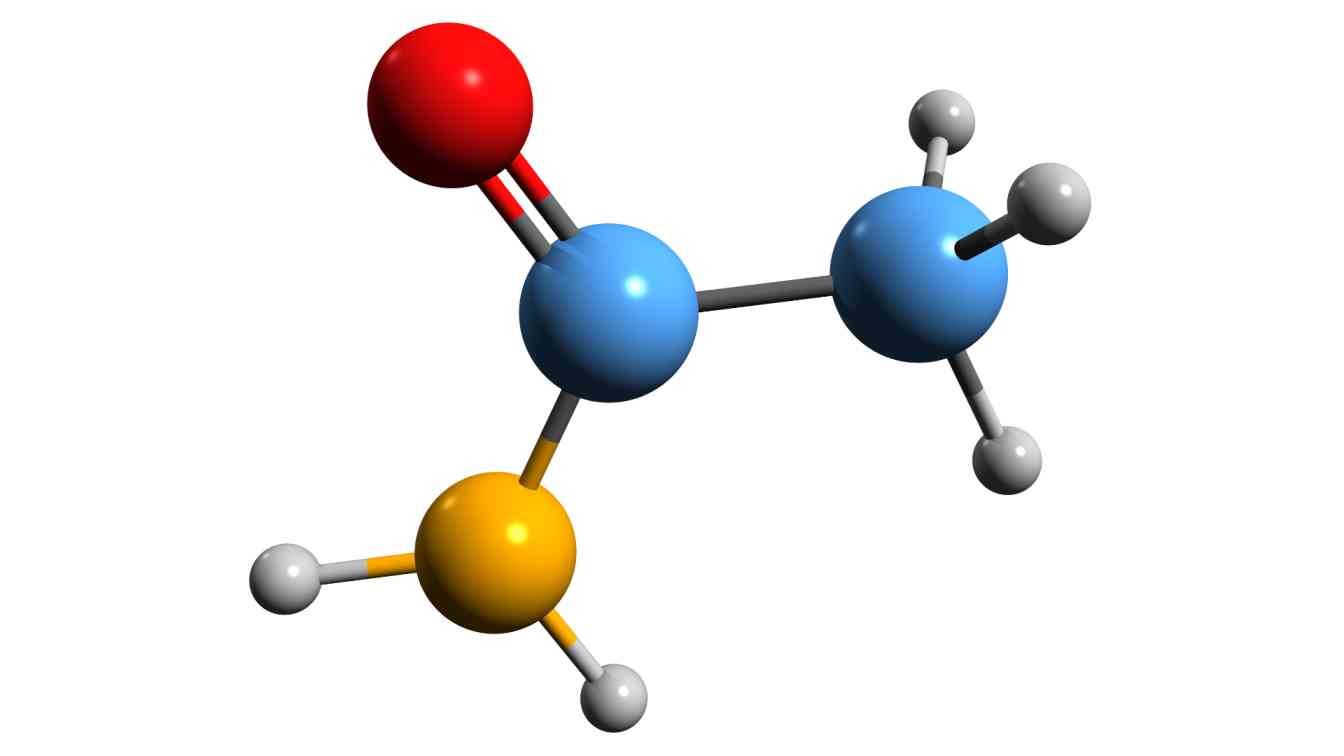
Chemical Structures - Lewis structures
Lewis structures, also known as Lewis dot diagrams or electron dot diagrams, are a way of representing the electron configuration and chemical bonding of a molecule or ion. They were developed by Gilbert N. Lewis in 1916 and are commonly used in organic chemistry, inorganic chemistry, and biochemistry.

In Lewis structures, the valence electrons of each atom in a molecule are represented by dots or lines. The dots represent individual electrons, while the lines represent pairs of shared electrons. Each dot or line is placed around the atomic symbol of the atom to indicate the number of valence electrons it possesses. The arrangement of the dots and lines also shows the connections between atoms in the molecule.
The steps to draw a Lewis structure are as follows:
- Determine the total number of valence electrons for all the atoms in the molecule.
- Identify the central atom in the molecule, which is usually the least electronegative element.
- Place the central atom in the centre of the diagram and connect it to the other atoms with single bonds.
- Place the remaining electrons around the atoms, starting with the outer atoms and filling the octet rule for each atom.
- Check that the number of electrons used equals the total number of valence electrons.
- If there are extra electrons, add them to the central atom as lone pairs.
- If there are not enough electrons, convert one or more of the lone pairs to a double or triple bond.
- Check that each atom has a full octet or duet.
Lewis structures are useful for predicting the properties of molecules and reactions. They provide a visual representation of the electron distribution in a molecule, which can help explain its chemical behaviour. These can also be used to determine the polarity of a molecule, which is important for understanding its interactions with other molecules.
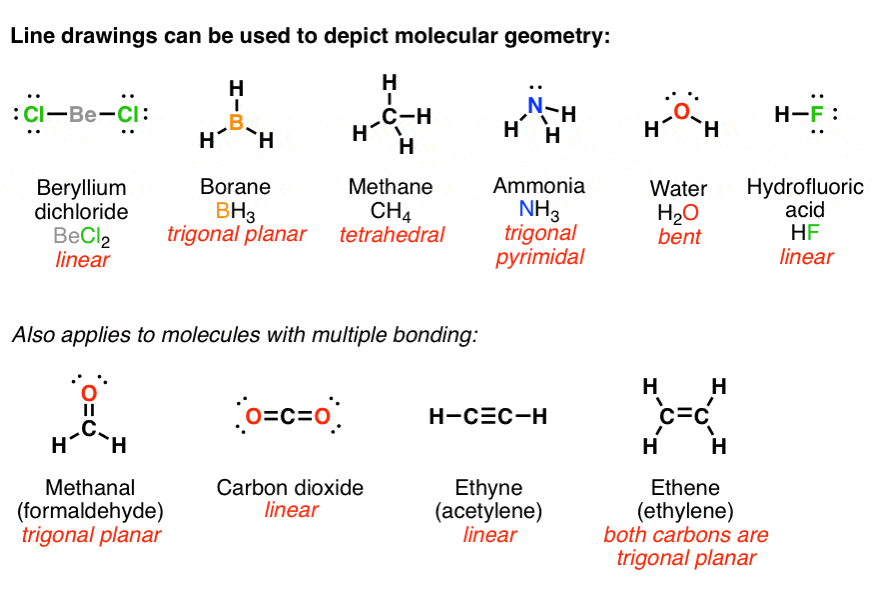
Lewis structures are particularly useful in organic chemistry for understanding the bonding and reactivity of organic molecules. In organic chemistry, carbon atoms are typically the central atoms in molecules, with other elements such as hydrogen, oxygen, nitrogen, and sulfur bonded to them.
In drawing Lewis structures for organic molecules, the following rules should be followed:
-
Carbon atoms always form four bonds, while hydrogen atoms form one bond.
-
Oxygen and nitrogen atoms form two bonds and two lone pairs of electrons.
-
Halogens (such as fluorine, chlorine, and bromine) form one bond and three lone pairs of electrons.
-
Charge is distributed so that the most electronegative atom (usually oxygen) carries the negative charge.
-
Multiple bonds (double or triple bonds) are used to satisfy the octet rule for atoms that require more than four bonds.
Lewis structures can be used to predict the reactivity of organic molecules. For example, in a nucleophilic substitution reaction, a nucleophile (an electron-rich species) attacks an electrophilic center (an electron-poor atom) in a molecule. By analyzing the Lewis structure of the molecule, it is possible to identify the electrophilic center and predict the site of nucleophilic attack.
Lewis structures can also be used to predict the acidity and basicity of organic molecules. In general, the more stable a molecule is, the less acidic it will be. By analyzing the Lewis structure of a molecule, it is possible to predict its stability and therefore its acidity or basicity.
Overall, Lewis structures are an essential tool for understanding the structure, bonding, and reactivity of organic molecules. They provide a visual representation of the electron distribution in a molecule, which is crucial for predicting its chemical behavior.
